The Client Story
A pharmaceutical company operating across five continents, with over 30 therapeutic lines and more than 12,000 ongoing trials, faced a direct compliance risk. Disjointed records stalled GenAI pilots, broke cross-trial reporting, and eroded trust. Without a centralized, governed data setup, both compliance and innovation were blocked. GroupBWT was brought in to design and implement a controlled data warehouse, built from scratch to handle regulatory pressure, enforce field-level traceability, and support analytical use cases at scale.
| Industry: | Pharma |
|---|---|
| Cooperation: | Since 2021 |
| Location: | Global |
“We had data everywhere. But no controlled version of the truth. GroupBWT helped us create one.” — VP, Data Strategy, Global R&D
“Now we answer audit queries in minutes—not rebuild reports every quarter.” — Director of Regulatory Intelligence
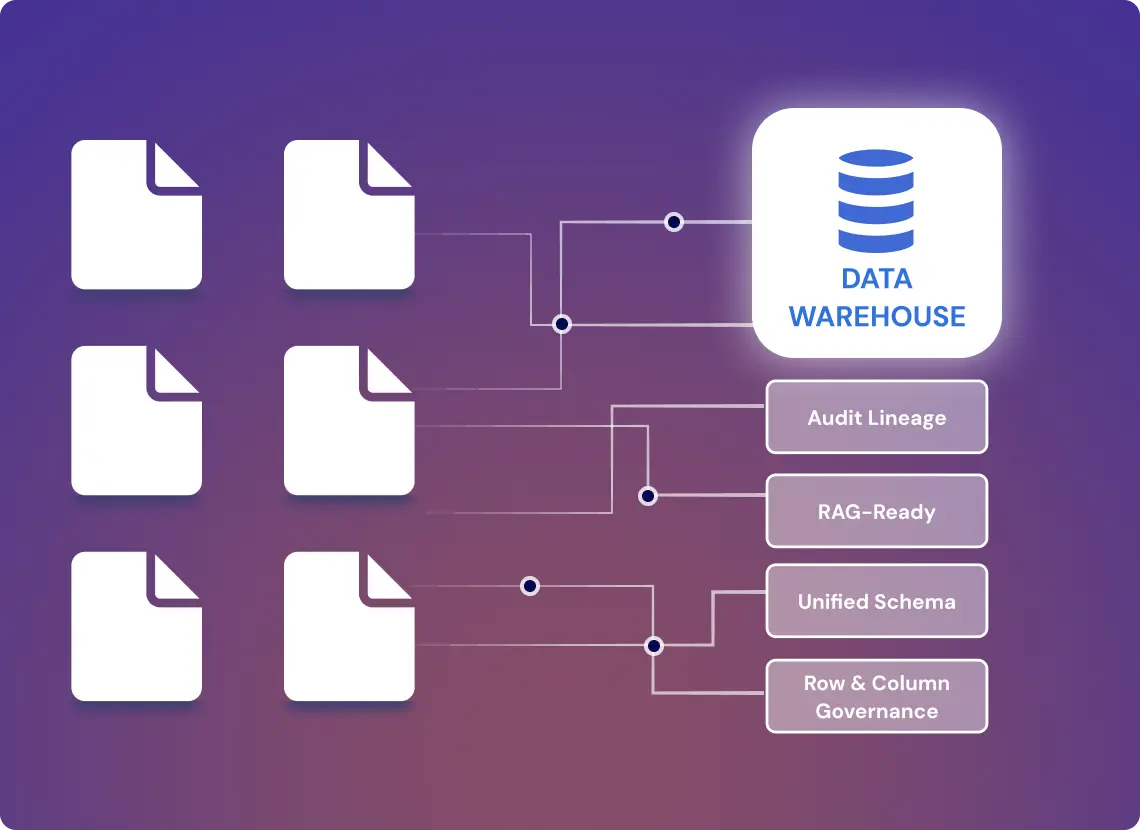
Map 22 Pharma Data Schemas into One Warehouse Backbone
Pharma teams manage fragmented data across PDF-based protocols, SAS (Statistical Analysis System) tables, CSV extracts, and logs from Electronic Data Capture (EDC) platforms. GroupBWT engineered a pharma-grade data warehouse ingesting from 7 pipelines, mapping 22 source schemas to a unified layer with full audit lineage.
The system ingests real-time data from Electronic Data Capture platforms, structures it using Clinical Data Interchange and Acquisition standards, and unifies views across research, safety, and regulatory teams. Governance blocks enable row- and column-level access for pharmacovigilance, clinical ops, and regulatory teams. The system now fuels internal dashboards, auto-generates FDA safety summaries, and supports RAG-ready context exposure for future GenAI research agents.
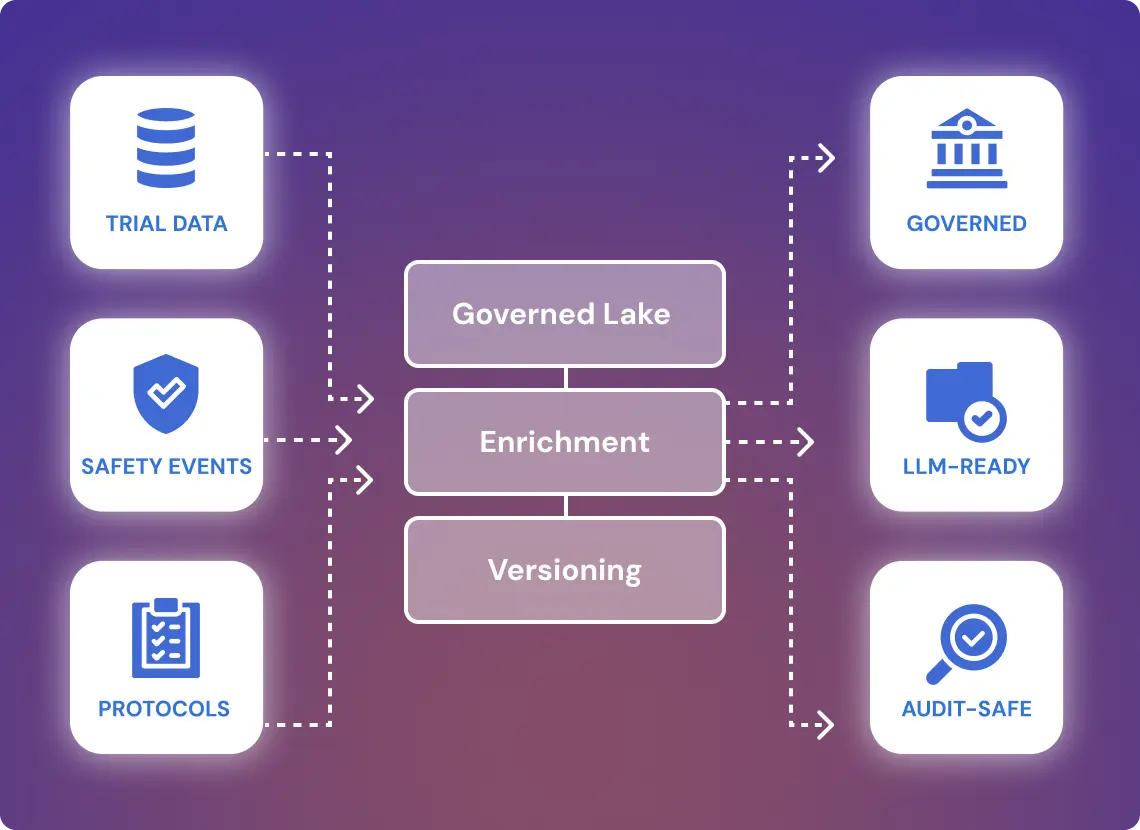
Modular Warehouse with Lineage & LLM Audit Readiness
GroupBWT designed a modular data warehousing stack using a lake-to-warehouse model. Raw data from trial systems, MedDRA-coded safety events, and protocol trackers were streamed into a governed lake layer, enriched, and versioned before warehouse transformation. The warehouse was deployed on a hybrid Postgres/Snowflake stack, with DBT-based modeling, dynamic masking, and historical record tracing.
LLM readiness was built in: vector metadata and document embeddings were generated for trial arms, adverse events, and investigator profiles. All transformations were logged, tested, and monitored, with lineage dashboards for internal QA and regulatory auditors.
The goal wasn’t just to centralize data—it was to make every record usable, explainable, and safe to act on. That’s what pharma teams mandate.

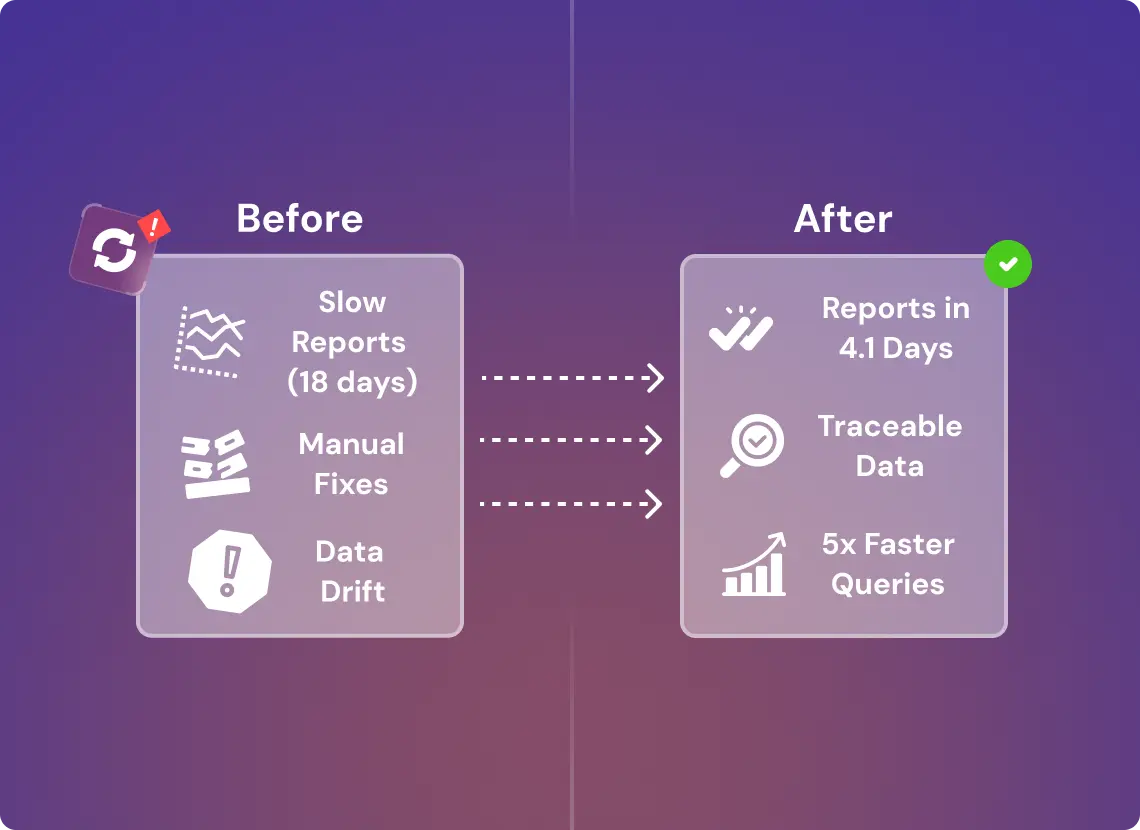
Cut Reporting Lag 70%, Boost Queries 5x, Zero Schema Drift
Reporting time on safety summaries dropped from 18.2 days to 4.1 days. Analysts gained consistent access to normalized, queryable data across domains. Cross-functional data issues—like inconsistent trial IDs or MedDRA code versions—were automatically flagged via warehouse QA checks. Performance improved by 5x across key reports (arm efficacy, adverse trend clustering). Every data point became traceable by source, timestamp, and transformation logic. LLM pilots for protocol Q&A and safety signal validation are now running on a traceable, warehouse-fed retrieval layer.
Pharma Warehouse with LLM Readiness and Full Audit Trail
GroupBWT designs pharma-grade data systems—from ingestion to insight. We integrate trial, safety, and regulatory data into governed warehouses, built for compliance, speed, and AI-readiness.
Let’s map your data flow and make it warehouse-ready.
Ready to discuss your idea?
Our team of experts will find and implement the best eCommerce solution for your business. Drop us a line, and we will be back to you within 12 hours.
You have an idea?
We handle all the rest.
How can we help you?



